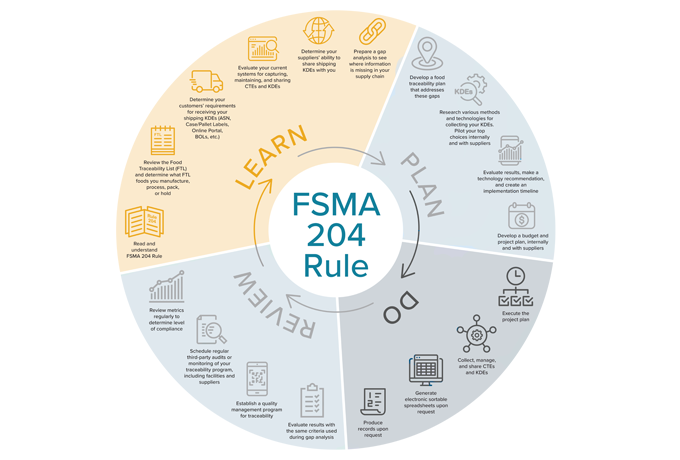
Timeline to FSMA 204 Compliance, Part 1: LEARN About FSMA 204
September 8, 2023
With just over two years until the FDA Food Traceability Rule enforcement date, your organization should already be thinking about its path to compliance.
But, you don’t have to be ready to comply overnight. Andy Kennedy, Principal Traceability Advisor at New Era Partners and co-writer of FSMA 204, designed a timeline outlining the path to compliance.
Andy recommends learning about the rule before you act and implement any new processes or systems.
1. Read and Understand Rule 204.
Supply Chain Role this applies to: Grower-Packer-Shipper, Processor, Distributor, Grocery Retail & Foodservice
The text of the final rule is long (179 pages!) and overwhelming. Andy used his intimate knowledge of the rule to put together a guide to reading the rule, which might be a helpful tool in getting started. As you read it, it’s important to understand the following:
- Who is subject to the rule?
- Definitions of terms commonly used throughout the text of the rule.
- What a Traceability Plan is, and that you must have one.
- The data records required from each Critical Tracking Event that applies to your business.
- The importance of securely storing and maintaining those records and keeping them accessible.
2. Review the Food Traceability List (FTL) and determine what FTL foods you manufacture, process, pack, or hold.
Supply Chain Role this applies to: Grower-Packer-Shipper, Processor, Distributor, Grocery Retail & Foodservice
Once you’ve read the rule and understand its requirements, you’ll want to determine if it applies to you. You’ll be subject to the rule’s requirements if you manufacture, process, pack, or hold any foods on the FTL. You’ll only be required to keep records for foods on the FTL, but you may consider keeping records for all foods your organization manufactures, processes, packs, or holds. The FDA has noted that it will review the list every five years, so while some foods are not on the list now, they may be eventually.
You’ll also want to understand the intricacies of the FTL foods. You may produce an ingredient not on the FTL in its raw form, but if it goes into an FTL food, then records may be required.
3. Determine your customers’ requirements for receiving your shipping KDEs (ASN, Case/Pallet Labels, Online Portal, BOLs, etc.).
Supply Chain Role this applies to: Grower-Packer-Shipper, Processor, Distributor
If your customers don’t have requirements in place, be proactive and ask them what they plan to do to ensure FSMA 204 compliance. Start by asking:
- Will they require an ASN (Advanced Shipping Notice)?
- Will they require PTI-compliant case labels on all cases and SSCC labels on all pallets?
- Can their system ingest data from a flat file (.csv), or will they require you to enter data into an online portal or have a cloud-based solution of your own?
- Are they planning to implement a network traceability solution across their entire supply chain? If so, will your organization be onboarded as part of their network implementation, or will you be expected to enroll and implement the system on your own?
If you know what will be required of your organization, you can ensure that their requirements fit into your own processes. By starting your own path to compliance sooner than later, you can avoid costly investments that may be irrelevant if your trading partners require other methods of sharing KDEs.
4. Determine your suppliers’ ability to share shipping KDEs with you.
Supply Chain Role this applies to: Processor, Distributor, Grocery Retail & Foodservice
Are you receiving KDEs from your supplier in a readily accessible format? When considering your own traceability processes and your customers’ requirements, you’ll also want to be sure your suppliers are sending you what you need in such a format that your systems can quickly ingest that data. Below are two steps we recommend to determine your suppliers’ ability to share shipping KDEs with you.
1. Audit Your Suppliers’ Labeling Practices
Start recording information about the labels on cases your suppliers send you. Observe:
- Whether or not every case contains a label and if it is scannable
- If the information on the label covers most of the shipping/receiving KDEs, and if a system is in place to capture KDEs not included on the label.
Did you notice any significant gaps in information? Are the labels in a format that is easy to ingest? Keep in mind that scanning a GS1-128 barcode is much more efficient than manually typing the same information into a computer.
2. Determine How You Will Ingest Your Suppliers’ Data
Getting the data you need from suppliers is only half the battle. You must also be able to ingest and access that data in your own system, which you can do in a variety of ways:
- Import a flat file with your suppliers’ data into your own system. This method offers limited visibility and will make it challenging to gather the data into an electronic spreadsheet within 24 hours of an FDA request. Paper records also lack the data insights that online platforms provide.
- Use an Advance Ship Notice (ASN) to gather data ahead of a shipment.
- Use an Application Programming Interface (API) to exchange data between your system and your suppliers’ systems.
- Ask your suppliers to use traceability software that stores shipping KDEs in the cloud and makes the data accessible to you.
5. Evaluate your current systems for capturing, maintaining, and sharing CTEs and KDEs.
Supply Chain Role this applies to: Grower-Packer-Shipper, Processor, Distributor, Grocery Retail & Foodservice
You’ll likely want to adopt the same system for capturing and maintaining your own KDEs as you will for sharing them.
While FDA does not require that you use certain solution providers or even that you use a digital, cloud-based solution, it’s important to keep in mind that master and event data records must be made available in an electronic sortable spreadsheet, along with any information needed to understand those records, within 24 hours of the FDA’s request. Larger organizations may find this 24-hour turnaround impossible if records are captured and maintained on paper or in multiple spreadsheets.
A network traceability solution is a good option for enterprise retail and foodservice organizations. Implementing a cloud-based solution across your supplier network can help you gain a complete picture of your supplier network to instantly traceforward and traceback to the traceability lot code. You’ll be able to more easily:
- Enable suppliers to share KDEs with you via the method that fits their operations.
- Gain an instant overview of your supplier network and verify compliance with your requirements.
- Identify orders with missing KDEs to outline an improvement plan.
- Access a comprehensive view of all traceability data received for all orders from your supplier network.
- Quickly generate your electronic sortable spreadsheet.
Learn how the iFoodDS Network Traceability Solution (PDF Download) can help your enterprise organization confidently capture, maintain, and share KDEs for compliance with FSMA 204.
6. Prepare a gap analysis to see where information is missing in your supply chain.
Now that you’ve:
- Audited your supply chain partners;
- Evaluated your own systems, and;
- Considered required updates to your processes and systems
You should have enough information to prepare a detailed gap analysis outlining the following:
- How your processes fit your customers’ expectations and how you may need to adjust.
- Whether your supply chain partners are sending you the required KDEs and your ability to ingest them.
- Whether or not your current systems enable you and your supply chain partners to fully comply with FSMA 204.
The Next Step: Planning
Now, it’s time to PLAN. Work with your supply chain partners to ensure your planned processes align. Start reviewing different traceability solutions that fit your organization’s needs and your customers’ requirements.
View our FSMA 204 timeline to better understand what it takes to plan and prepare your organization for FSMA 204 compliance. iFoodDS is the leader in food supply chain solutions. Request a consultation to learn how we can help guide your organization through FSMA 204 compliance.
Read our complete Timeline to FSMA 204 Compliance Series:
- Part 1: Learn (read this article)
- Part 2: Plan
- Part 3: Do
- Part 4: Review