Timeline to FSMA 204 Compliance, Part 2: PLAN For FSMA 204
September 18, 2023
With just over two years until the FDA Food Traceability Rule enforcement date, your organization should be starting to formulate a plan to comply with the rule.
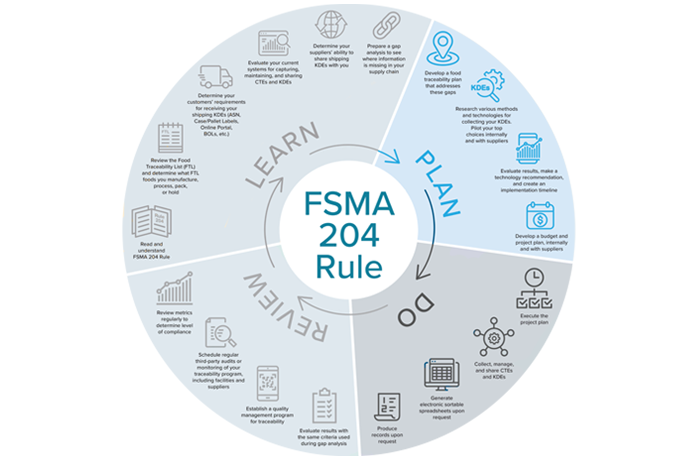
Andy Kennedy of New Era Partners, and co-writer of FSMA Rule 204, designed a timeline outlining the path to compliance. He recommends first LEARNING about FSMA 204 and before you act and implement any new processes or systems, then PLANNING your path to compliance.
1. Develop a food traceability plan that addresses your process and system gaps
Supply Chain Role this applies to: Processor, Distributor, Grocery Retail & Foodservice
FDA requires a Food Traceability Plan with specific requirements as to what you need to include. We’ve outlined those requirements here and provided an example of a Traceability Plan in this downloadable infographic.
Before creating the Traceability Plan as required by FDA, you’ll want to think through your organization’s traceability process for complying with FSMA 204. In the learning phase, you identified gaps in your systems and processes, and in those of your supply chain partners. Now is the time to address those gaps.
Start by asking:
- Are your systems capable of capturing, storing, and sharing key data elements (KDEs)?
- Are your trading partners sending, or able to send, all required shipping KDEs?
- Are you able to ingest KDEs from your trading partners?
- Do your customers have data-sharing requirements that you’re not able to meet?
- Can you create an electronic sortable spreadsheet with the FSMA 204-required records within 24 hours?
- What else might your organization be missing?
Once you’ve identified gaps in your process, build out a plan for addressing those gaps. Be sure you include your trading partners in your planning process. Their processes and methods for sharing data must align with yours in some fashion so that data-sharing is possible.
2. Research various methods and technologies for collecting your KDEs. Pilot your top choices internally and with suppliers.
Supply Chain Role this applies to: Processor, Distributor, Grocery Retail & Foodservice
The FDA’s Food Traceability Rule does not require anyone to use one specific solution, but you do need to be able to capture, store, and share KDEs with your trading partners. We recommend a secure, cloud-based solution, but sharing data is not a “one-size-fits-all” for suppliers of all sizes and operations, and it’s okay to use different systems and tools. For example:
1. Implement a cloud-based traceability solution that stores KDEs in the cloud, and talk to your suppliers about doing the same.
With a cloud-based traceability solution, you can easily capture, store and share KDEs with your trading partners. This method is critical for larger enterprise organizations with complex supply chains. Data is readily available when needed to create your electronic sortable spreadsheet; plus, you’ll gain visibility into your supply chain, enabling you to more easily notice areas of concern and address them before an audit, recall, or outbreak occurs.
Note that not all solution providers are equal, and you’ll want to ensure they meet the minimum solution provider requirements that will enable you to comply with FSMA 204.
To learn more, see our solution provider checklist for guidance.
2. Use Advanced Shipping Notices (ASNs) to gather data ahead of a shipment.
This electronic format (known as an electronic data interchange, or EDI) makes sending the information easy and quick. Other benefits include accurate transfer of data, improved inventory management, and warehouse efficiency. Note that ASNs only support shipping KDEs. You may need to use other data-sharing and ingesting methods in addition to ASNs, depending on what Critical Tracking Events (CTEs) you are responsible for.
Not all suppliers have ASN capabilities or the ability or funds to implement them. We recently surveyed a large supplier network, and only 55% of 115 respondents said they have ASN capabilities. ASNs can be costly to put in place, so it’s important to offer your supplier options.
3. Use an Application Programming Interface (API) to exchange data between your system and your suppliers’ systems.
This method works well when dealing with disparate systems across your supply chain. As an enterprise organization, your supply chain is complex. It won’t be possible to get all your trading partners on the same system or even use systems that easily talk to each other. An API can be a great way to bridge those systems so that you can more easily share and capture traceability records.
4. Import a flat file (.csv) with your suppliers’ data into your own system.
If your supplier’s organization is small and manually capturing KDEs, then storing them in a spreadsheet and sharing via secure methods is manageable for you and works for your trading partners, then this could be an acceptable method.
Read our article “4 Myths About FSMA 204 Dispelled” to learn more about common misconceptions around data-sharing requirements.
Once you’ve narrowed your list of food traceability solution providers, ask if you can pilot the program internally and with a small group of trading partners. This will help you better understand the solution’s capabilities, ease of use, gaps, or red flags.
3. Evaluate results, make a technology recommendation, and create a traceability implementation timeline.
Supply Chain Role this applies to: Processor, Distributor, Grocery Retail & Foodservice
Once you’ve evaluated and piloted your top choice, it’s time to decide how to move forward. Make your recommendation to key stakeholders, sign the contract, and work with your new solution partner to develop an implementation timeline. Implementation within your organization and across your supply chain can take months, or even more than a year, so doing this early will alleviate undue stress, costs, or risk of non-compliance by the January 20, 2026 FSMA 204 compliance date.
When you’re ready to implement your new solution, look for a solution partner that clearly communicates expectations, timelines, and software requirements upfront. Awareness of these expectations early on will set you and your new solution partner up for a successful launch and a stronger ongoing relationship.
4. Develop a budget and project plan internally and with suppliers.
Supply Chain Role this applies to: Processor, Distributor, Grocery Retail & Foodservice
Now that you have selected a cloud-based food traceability solution or another method of capturing, maintaining, and sharing your traceability data, you should have a good idea of the associated costs and can finalize your budget.
You’ll also need to solidify your project plan, including implementation and ongoing use of your new solution. You’ll likely need to put new processes in place. Ideally, you’ll start to notice new areas of efficiency and new opportunities to use your data.
Conclusion
Now that you have a plan in place, it’s time to DO. Work with your internal teams and supply chain partners to take action on your agreed-upon processes.
To learn more about the overall planning for traceability, view the FSMA 204 timeline to better understand what it takes to take action. iFoodDS is the leader in food supply chain solutions. Request a consultation to learn how we can help guide your organization through FSMA 204 compliance.
Learn more about compliance with FDA’s Food Traceability Rule, FSMA 204, by visiting our FSMA 204 Information Hub.
Read our complete Timeline to FSMA 204 Compliance Series:
- Part 1: Learn
- Part 2: Plan (read this article)
- Part 3: Do
- Part 4: Review