How Processors Can Collect KDEs Efficiently
October 19, 2022
Note: This content has been updated to reflect the final version of FSMA Rule 204.
FSMA Rule 204 was finalized in November 2022 and will go into effect January 20, 2026. The Rule mandates the collection of Key Data Elements (KDEs) linked to traceability lots, among other requirements. This could prove challenging for processors given the complexity of the processing environment. Receiving the right data from your suppliers is critical.
This article will focus solely on transformation KDEs and provide ideas for efficiently capturing them. Note that most processors will need to record shipping and receiving KDEs as well. Please consult our guide to receiving and shipping KDEs for more information.
The Transformation Critical Tracking Event
Transformation is defined as, “an event in a food’s supply chain that involves manufacturing/processing or changing a food (e.g., by commingling, repacking, or relabeling) or its packaging or packing, when the output is a food on the Food Traceability List (FTL).” The FDA further clarifies that “Transformation does not include the initial packing of a food or activities preceding that event (e.g., harvesting, cooling).” A notable change in the final Rule is that the FDA combined the old creation CTE with transformation.
Transformers must establish and maintain records containing and linking the new traceability lot code for the food produced through transformation to these KDEs. Note that the FDA lays out two different processes that fall under transformation – transformation where foods on the Food Traceability List (FTL) are used as ingredients, and transformation where the output is a food on the FTL. Each process has its own set of KDEs.
Transformation KDEs for FTL foods used as ingredients:
- Traceability lot code for the food
- Product description for the food to which the traceability lot code applies
- For each traceability lot used, the quantity and unit of measure of the food used from that lot
Transformation KDEs for a new FTL food produced:
- New traceability lot code for the food
- Location description for where you transformed the food (i.e., the traceability lot code source), and (if applicable) the traceability lot code source reference
- Date transformation was completed
- Product description for the food
- Quantity and unit of measure of the food
- Reference document type and reference document number
The Challenge of Recording KDEs in the Processing Environment
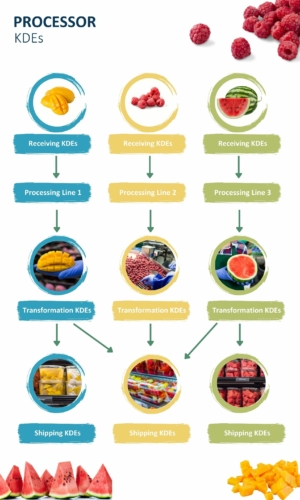
Processing environments pose a unique challenge for traceability recordkeeping. Processors must keep track of all the lots associated with raw ingredients and connect them to the finished product. In the event of an investigation, they must be able to traceback to the individual lots that went into a product. If there is a recall, they need to traceforward to identify every product that contains the recalled lot. Reference our illustration below to see just how complex KDE collection can be for processors.
NOTE: Click on the image to open a larger version.
How Processors Can Record KDEs Efficiently
As the above example illustrates, processing is so complex that processors need to have a traceability platform that can keep track of all the different lots and link raw ingredients to finished products. Whether that is your current ERP/WMS or a dedicated platform that specializes in traceability, you need to keep everything organized in a digital dashboard to ensure you’re collecting all the required KDEs and can easily access them after an FDA request.
Here are some key features you’ll need:
- Instant traceback/traceforward to help with outbreak investigations or recalls.
- The ability to link raw ingredients and finished products.
- Secure cloud-based storage to allow for access on multiple devices and across multiple user accounts in your organization.
- Visuals to help track supplier compliance, such as analytics and dashboards.
Collaboration With Your Suppliers is Critical
It’s important to note that recording your KDEs is not as simple as just investing in a traceability platform. You need to work with your suppliers so that you can capture the KDEs that you need.
Consider taking a collaborative approach to Rule 204, rather than leaving suppliers to navigate compliance on their own. Proactively vet different traceability solutions that would allow your suppliers to send you their data in a format that works for both of you. By providing solutions instead of imposing requirements, you’ll find your suppliers are more willing to work with you. You’ll not only get the right information, but also strengthen relationships.
Give Your Suppliers Multiple Options for Sharing Data
Depending on your traceability platform provider, you may be able to offer multiple ways to ingest your suppliers’ data, such as:
- Secure file transfer to accept a flat file (e.g., Excel or CSV) exported from your suppliers’ ERP systems
- EDI to accept ASNs
- API to exchange data between platforms
This goes back to what we said earlier about working with your suppliers to provide solutions. The more options your suppliers have for sending you their data, the more likely it is that they will do so.
Confirm Physical Products Match Electronic Records By Using Barcodes
Ask your suppliers to use GS1-128 barcodes on every shipment they send you so that you can easily verify each shipment matches the electronic traceability records you have on file. For example, let’s say you’re receiving shipments from suppliers A, B and C, and A uses a traceability solution, B uses an ASN, and C sends you a CSV file. All 3 use GS1-128 barcodes, so even though data was sent in different formats, all enable you to verify the physical product.
Work with iFoodDS to Get Your Entire Supplier Network in Compliance
iFoodDS understands the complexity of the food supply chain and the unique challenges of the processing environment. Our iFoodDS Trace Exchange™ (with IBM Food Trust™) solution was developed with the needs of complex supplier networks in mind. Our team provides the support you need to onboard your whole supplier network and track compliance. We enable instant traceback/traceforward and provide secure, cloud-based storage. Explore more features or request a consultation with our team.
This material is for informational purposes only and not for the purpose of providing legal advice. You should contact your attorney to obtain advice with respect to any particular issue or problem.